
Study for Adolescents (12-17 Years Old) Suffering from Primary Generalized Tonic-Clonic Seizures
Is your child suffering from primary generalized tonic-clonic seizures and between the ages of 12 and 17?
If so, your child may qualify for a clinical trial being conducted by SK Life Science studying an investigational medicine to evaluate efficacy and safety for the treatment of primary generalized tonic-clonic (PGTC) seizures for patients ages 12 to 17 years old.
What are primary generalized tonic-clonic seizures?
Primary generalized tonic-clonic seizures are also called convulsions and are what most people think of when they hear the word “seizure.” An older term for this type of seizure is “grand mal.” They are the most common type of generalized seizure, or seizure that affects the whole body. These seizures cause violent muscle contractions and loss of consciousness.
The muscles of the arms and legs stiffen during the first part of the seizure, called the tonic part (tonic means stiffening). During the tonic phase air being forced past the vocal cords can cause a cry or groan. The person may lose consciousness and fall to the floor. Also, a person may bite their tongue or inside of their cheek. If this happens, saliva may look a bit bloody.
Then, the muscles of the arms, legs and face begin to jerk during the second phase of the seizure, known as the clonic part (clonic means rhythmical jerking).
During the clonic phase, the arms and usually the legs begin to jerk rapidly and rhythmically, bending and relaxing at the elbows, hips, and knees. After a few minutes, the jerking slows and stops. The person’s face may look dusky or a bit blue if they are having trouble breathing or the seizure lasts too long. The person may also lose control of their bladder or bowel as the body relaxes. Consciousness, or a person’s awareness, returns slowly.
Some patients may only experience one part of the seizure. Others may experience both. Tonic-clonic seizures usually last between one and three minutes. Afterwards, the person may be sleepy, confused, irritable, or depressed.
What part of the brain is involved?
Tonic-clonic seizures can start in one or both sides of the brain.
When they start in both sides of the brain, they are called generalized tonic-clonic seizures or generalized onset motor seizures. Both terms mean the same thing.
What is the drug being investigated?
The drug being investigated in this study is cenobamate. Cenobamate, which is taken by mouth, belongs to a class of medicines called antiseizure. It acts in the brain to prevent seizures.
Cenobamate was approved for medical use in the United States in November 2019 for the treatment of partial-onset seizures in adults (18 years of age and older). In the European Union, it was approved in March 2021 for the adjunctive treatment of focal-onset seizures with or without secondary generalization in adults (18 years of age and older) with epilepsy who have not been adequately controlled despite a history of treatment with at least two antiseizure medications.
What do I need to know about the study?
The PGTC Study will involve up to 170 participants at up to 100 clinical sites worldwide.
The study will include:
- 12-week pre-randomization/screening period
- 22-week treatment period (neither the participant nor the investigator will be aware if the subject is being treated with drug or placebo while on existing antiseizure medications)
Participants in the study will receive the study medicine, tests and assessments, and attend study related visits at no cost. Participants can stop taking part in the clinical trial at any time without giving a reason. Caregivers of participants may also be reimbursed for some study-related expenses, such as costs associated with travel and hotels.
Eligible patients will be offered the ability to continue treatment with the investigational medicine following completion of the trial by enrolling in the follow-up open-label study; an open-label study allows investigators to continue evaluating the safety and efficacy of the investigational medicine after the primary study period has been completed.
Is my child eligible to participate in the study?
Here is a list of key Inclusion/Exclusion Criteria to participate in the study:
KEY INCLUSION CRITERIA
- Participant needs to be between 12 to 17 years old
- PGTC diagnosis (with or without other subtypes of generalized seizures) in the setting of idiopathic generalized epilepsy with at least 1.5-2 seizures/month
KEY EXCLUSION CRITERIA
- Participant cannot have a diagnosis of partial onset seizure
- Participant cannot have a diagnosis of Lennox-Gastaut Syndrome
- Participant cannot have a history of any serious drug-induced hypersensitivity reaction
- Participant cannot have clinically significant abnormalities or disease that, in the opinion of the principal investigator, could affect the participant’s safety or conduct of the study
Frequently Asked Questions
What is a study?
Why are studies important?
What is the purpose of the PGTC study?
Will compensation for time and travel be provided?
Is there a cost to participate?
- Your child will receive study-related care from a team of experienced doctors and nurses throughout the study.
- All study-related visits, tests, assessments, and investigational medication will be provided at no cost to you.
What else do I need to consider?
Where are the study sites located?
Investigator Locator
Contact the site closest to you to learn if your child is eligible.
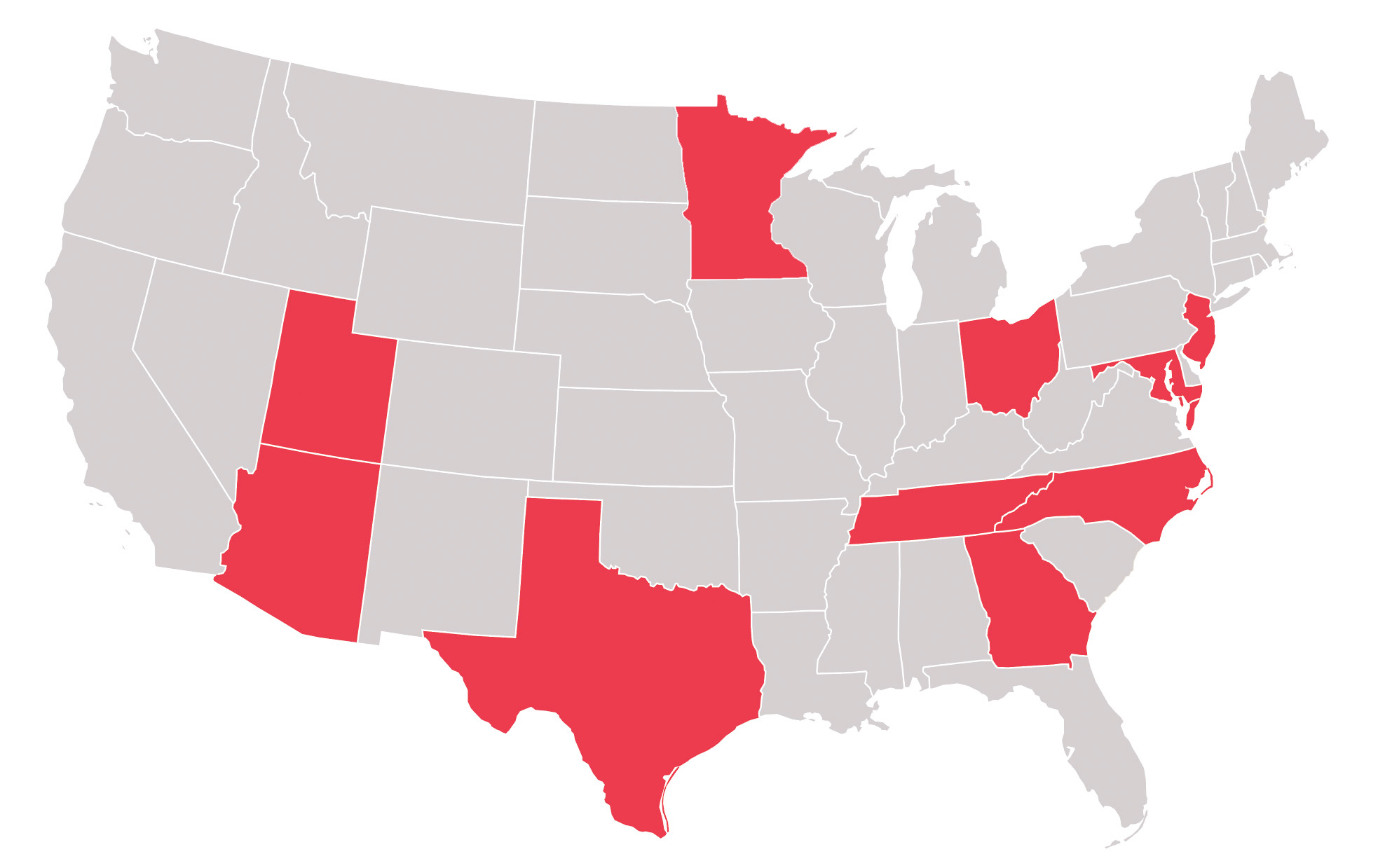
There are study sites located in the United States as well as Australia, Germany, Hungary, Poland, Slovakia, South Korea and Spain View the study sites by location to determine which is closest to you.
The Study
Is Taking
Place Now!
Northeast Regional Epilepsy Group (NEREG)
SITE CONTACTS:
20 Prospect Ave, Suite 800/801
Hackensack, NJ 07601
Eric Segal
Principal Investigator
Hardik Rana, MBBS
Telephone: +1 551-497-5000
Email: hrana@epilepsygroup.com
The Children’s Hospital at Saint Peter’s University Hospital
SITE CONTACTS:
254 Easton Avenue
New Brunswick, NJ 08901
Carlos Lastra
Principal Investigator
Shona McMahan
Research Nurse Coordinator
Telephone: +1 732-745-8600 ext: 6096
Email: smcmahan@saintpetersuh.com
University of Toledo HSC
3000 Arlington Ave Mail Stop 1083
Toledo, Ohio 43614
SITE CONTACTS:
Naeem Mahfooz
Principal Investigator
Stephanie Wilson MSN, APRN, CCRC
Study Coordinator
Telephone: +1 419-383-6721
Email: stephanie.wilson@utoledo.edu
Le Bonheur Children's Hospital
848 Adams Avenue, Memphis, TN 38103
SITE CONTACTS:
James Wheless
Principal Investigator
Sarah Barve
Telephone: +1 901-287-7467
Email: sarah.barve@lebonheur.org
Pediatrix Neurology, Austin
7940 Shoal Creek Blvd Ste 100
Austin, TX 78757
SITE CONTACTS:
Karen Keough
Principal Investigator
Victoria Henderson and Talitha Durgin
Study Coordinators
Telephone: +1 737-443-5194
Email: victoria.henderson@pediatrix.com
Center For Neurosciences
2450 E. River Rd Tucson, AZ 85718
SITE CONTACTS:
Yeeck Sim
Principal Investigator
Christina Diaz
Research Coordinator
Telephone: +1 520-795-7750 ext 7817
Email: CDiaz@neurotucson.com
(U HEALTH) University of Utah Clinical Trials Office
295 Chipeta Way, Suite 1S100
Salt Lake City, UT 84108
SITE CONTACTS:
Matthew Sweney
Principal Investigator
Hina Yazdani, CCRC
Telephone: +1 801-631-1067
Email: hina.yazdani@hsc.utah.edu
Clinical Integrative Research Center of Atlanta/PANDA Neurology
5887 Glenridge Dr. Suite 150
Atlanta, GA 30328
SITE CONTACTS:
Robert J. Flamini
Principal Investigator
Cristy Miles
Study Coordinator
Telephone: +1 678-528-0961
Email: cmiles@pandaneurology.com
Children's Healthcare or Atlanta
2174 N. Druid Hills Rd Atlanta, GA 30329
SITE CONTACTS:
Bryan Philbrook
Principal Investigator
Zamzam Kassim
Senior Research Coordinator
Telephone: +1 404-785-2320
Email: Zamzam.Kassim@choa.org
Mid-Atlantic Epilepsy and Sleep Center
6410 Rockledge drive, Suite 610
Bethesda, MD 20817
SITE CONTACTS:
Pavel Klein
Principal Investigator
Salman Hashmi
Study Coordinator
Telephone: +1 301-530-9744
Email: hashmis@epilepsydc.com
Duke Clinical Research
SITE CONTACTS:
Muhammad Zafar
Principal Investigator
Kay Doukellis
Clinical Research Nurse Coordinator
Email: Katheryn.doukellis@duke.edu
Mayo Clinic
Rochester, MN 55905
SITE CONTACTS:
Lily Wong-Kisiel
Principal Investigator
Precilla Ruiz
Coordinator
Telephone: +1 507-538-6606
Email: Ruiz.precylla@mayo.edu
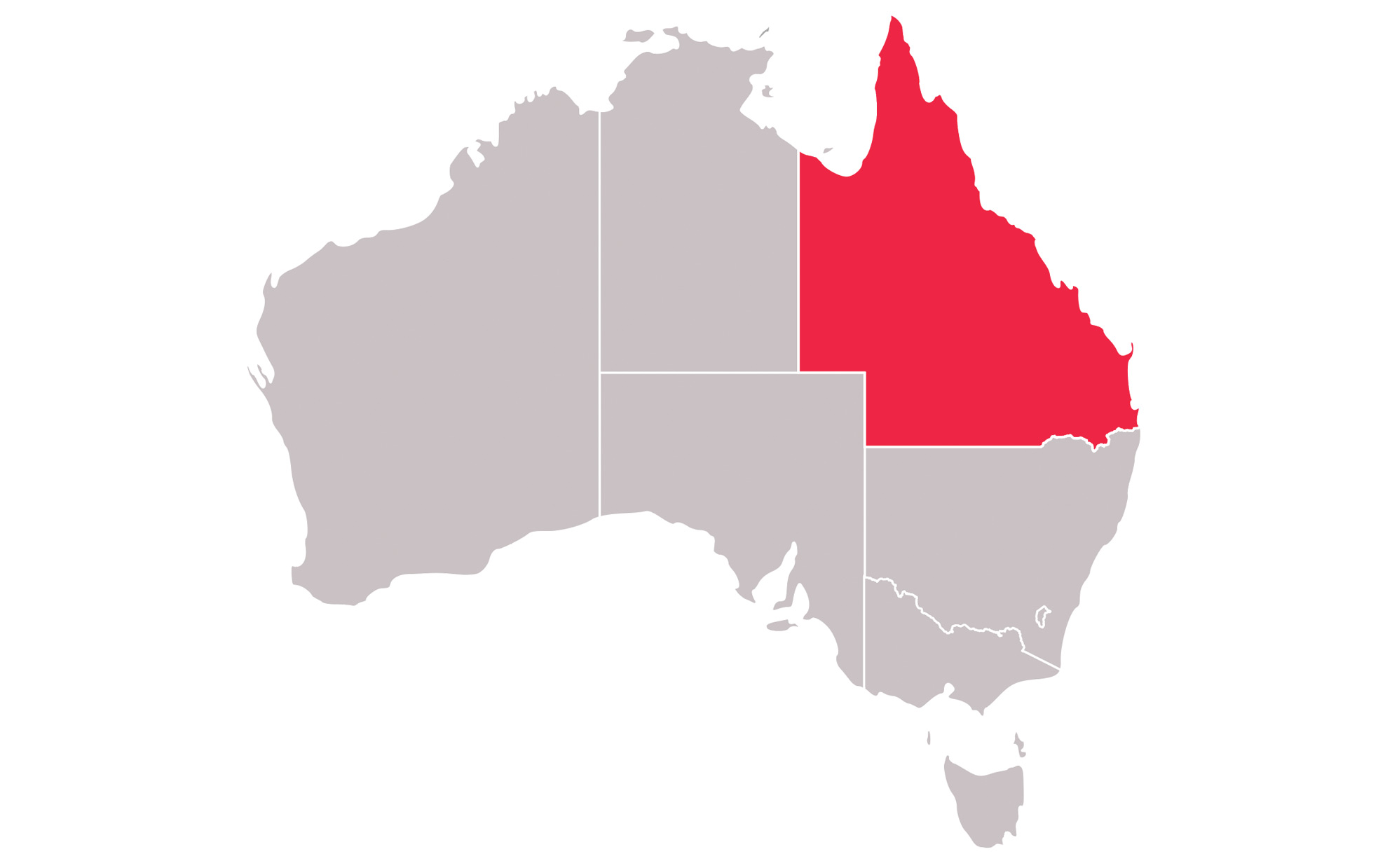
Queensland Children’s Hospital
SITE CONTACTS:
Dr. Catherine (Kate) Riney
Email:
epilepsy-research@health.qld.gov.au
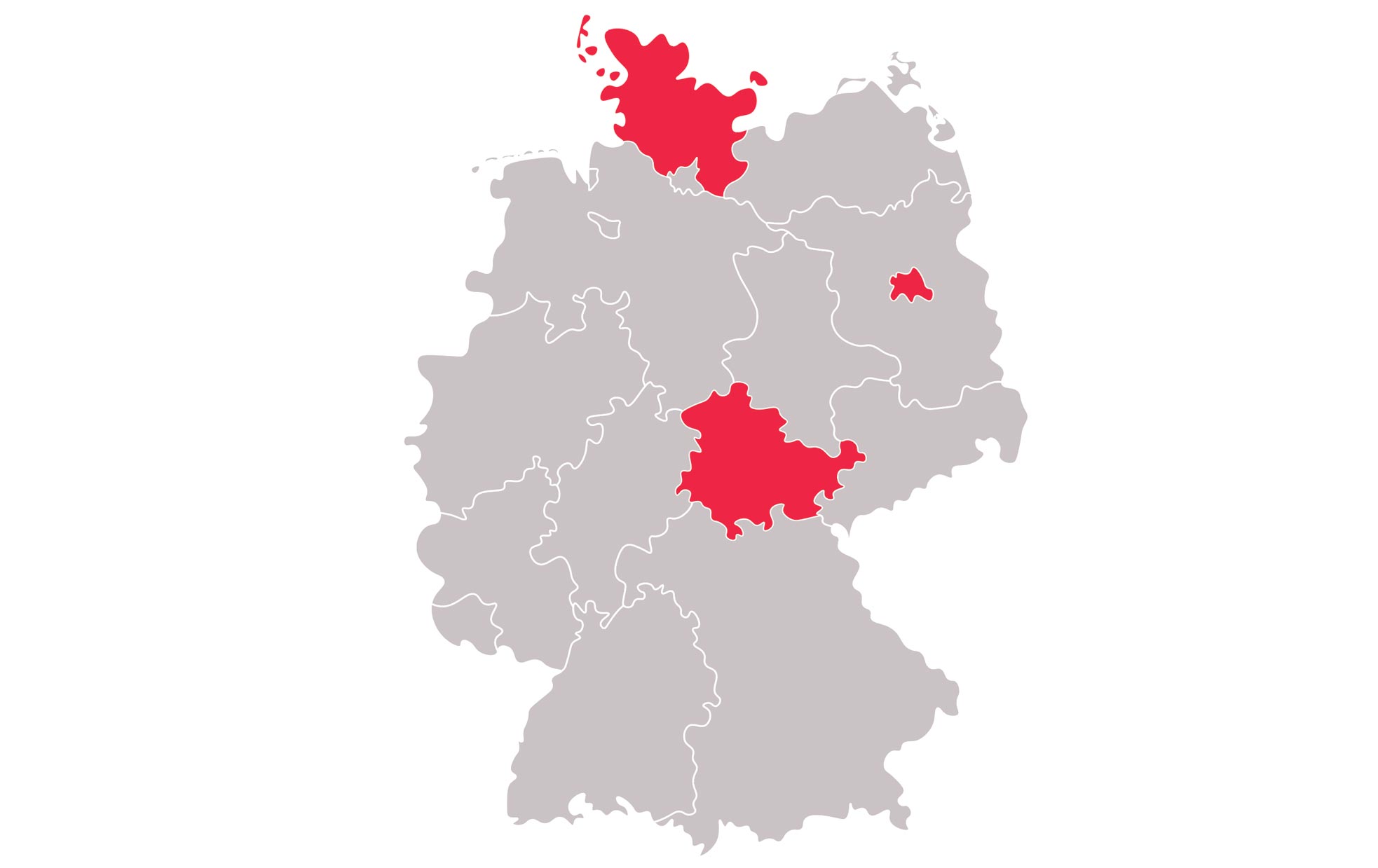
Universitaetsklinikum Schleswig-Holstein - Campus Kiel
Klinik für Neuropädiatrie Kiel, SCHLESWIG-HOLSTEIN 24105
SITE CONTACTS:
Hiltrud Muhle
Principal Investigator
Irene Lehman
Email: Irene.Lehman@uksh.de
Universitätsklinikum Jena
Am Klinikum 1 Jena, THURINGEN 07747
SITE CONTACTS:
Ralf Husain
Principal Investigator
Anja Mueller
Telephone: +49 3641 9329690
Email: anja.mueller@med.uni-jena.de
Charite - Universitätsmedizin Berlin - Sozialpädiatrisches Zentrum
SITE CONTACTS:
Angela Kaindl
Principal Investigator
Email: neuropaed-studie@charite.de
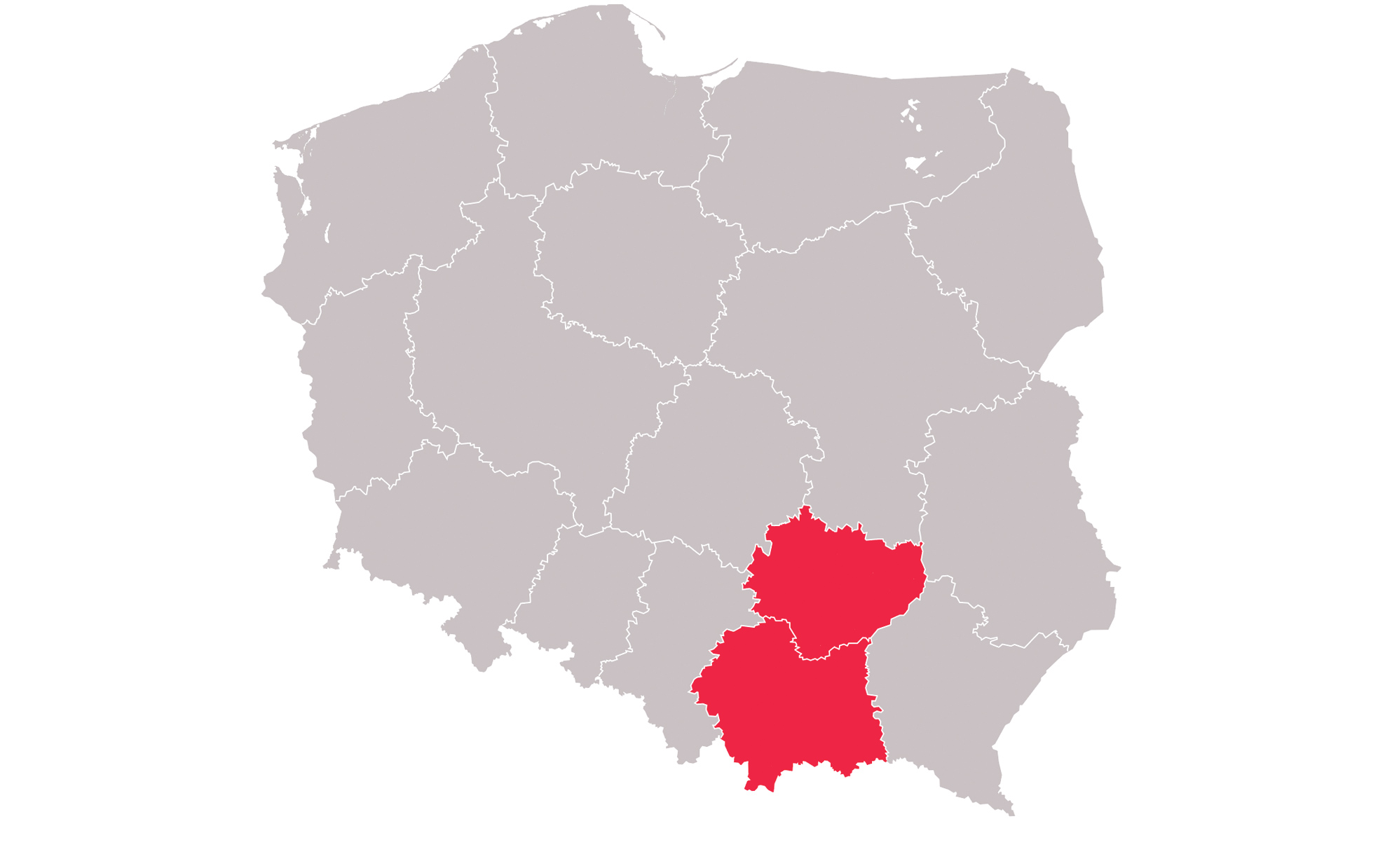
NZOZ - Centrum Neurologii Dziecięcej i Leczenia Padaczki
25-316 Kielce, Poland
SITE CONTACTS:
Anna Gniatkowska-Nowakowska
Principal Investigator
Telephone:
+48604 091 678
Email:
ankagn@mp.pl
Kraków, Poland
Marta Żołnowska
Principal Investigator
Telephone:
+48 12-353-55-55
+48 608 336 668
Email:
marta.zolnowska@gmail.com
IN MEDIC
SITE CONTACTS:
Jana Chamilova
Principal Investigator
Email:
jana.chamilova7@gmail.com
KONZÍLIUM
SITE CONTACTS:
Magdaléna Perichtová
Principal Investigator
Email:
konzilium.med@gmail.com
Chungbuk National University Hospital
776, 1sunhwan-ro, Seowon-gu, Cheongju-si, Chungcheongbuk-do, 28644, South
SITE CONTACTS:
Chae, Kyu Young
Principal Investigator
Email: a210287@chamc.co.kr
SMG-SNU Boramae Medical Center
20, Boramae-ro 5-gil, Dongjak-gu, 7061 Seoul, South Korea
SITE CONTACTS:
Choi, Ji-Eun
Principal Investigator
HyeJin Park
Telephone: +82-2-870-2375
Ajou University Hospital
164, World cup-ro, Yeongtong-gu, 16499 Suwon-si, Gyeonggi-do, South Korea
SITE CONTACTS:
Jung, Da-Eun
Principal Investigator
Jiyeon Ha
Phone: 82-31-219-4445